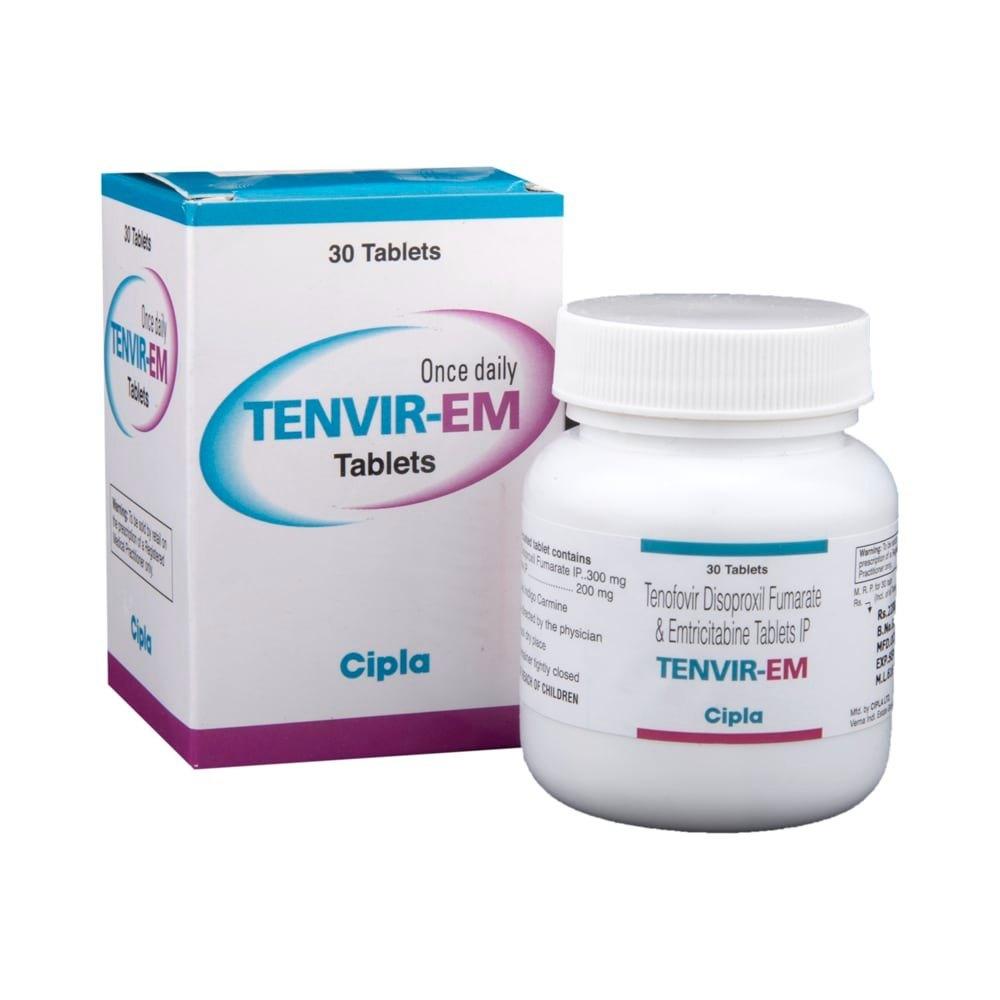
Tenvir EM, a prominent pharmaceutical combination of two active antiretroviral compounds: Emtricitabine and Tenofovir disoproxil fumarate, is crucial in the management and prevention of the Human Immunodeficiency Virus (HIV). This combination therapy, positioned at the forefront of modern antiretroviral treatments, delivers a potent intervention against HIV-1 infection.
Compound Synthesis and Structure
- Emtricitabine: Also known by its abbreviation FTC, Emtricitabine is a nucleoside reverse transcriptase inhibitor (NRTI). Its structure mimics that of the natural nucleoside cytidine, making it proficient in inhibiting the reverse transcriptase enzyme of HIV.
- Tenofovir disoproxil fumarate: This compound, abbreviated as TDF, belongs to a class of antiretroviral drugs called nucleotide reverse transcriptase inhibitors (NtRTIs). TDF acts as a prodrug, which means that post-ingestion, it undergoes metabolic conversion to produce its active form, Tenofovir diphosphate. This activated form then effectively competes with the natural substrate deoxyadenosine 5′-triphosphate, subsequently leading to termination of the DNA chain and inhibiting viral replication.
Mechanism of Action
When combined, Emtricitabine and Tenofovir work synergistically to provide a robust barrier against HIV replication. Their action focuses primarily on the inhibition of reverse transcriptase, an enzyme that is indispensable for the virus.
Upon entering the cell, both Emtricitabine and Tenofovir are transformed into their active triphosphate forms. These active entities incorporate themselves into the viral DNA, inducing premature termination of its synthesis. This process effectively curtails the virus’s capacity to reproduce and infect other cells.
Pharmacokinetics
Upon oral administration of Tenvir EM, both Emtricitabine and Tenofovir disoproxil fumarate demonstrate rapid absorption profiles. Here’s a detailed exploration:
- Absorption: Post ingestion, maximum plasma concentrations of Emtricitabine and Tenofovir are typically observed between 1-2 hours.
- Distribution: Both compounds are widely distributed across body compartments. They exhibit an ability to penetrate the central nervous system, thus providing therapeutic concentrations in cerebrospinal fluid.
- Metabolism: Emtricitabine undergoes minimal metabolism, with the majority being excreted unchanged in urine. On the other hand, Tenofovir disoproxil fumarate transforms to its active form, Tenofovir diphosphate, in cells.
- Excretion: Predominantly, Emtricitabine is expelled through renal excretion, whereas Tenofovir follows both renal and fecal pathways.
Indications
Tenvir EM serves a dual role in HIV management:
- Treatment: As part of combination therapy, Tenvir EM aids in the treatment of HIV-1 infection in adults and pediatric patients weighing at least 17 kg.
- Prevention: For those at high risk, Tenvir EM acts as a prophylactic, reducing the chances of acquiring HIV-1 infection. This preventive measure is commonly referred to as Pre-exposure Prophylaxis (PrEP).
Dosage and Administration
For Tenvir EM to be most effective, adherence to the recommended dosing schedule is imperative:
- Treatment of HIV-1 Infection: One tablet of Tenvir EM daily, in conjunction with other antiretroviral agents, is prescribed. It can be consumed with or without food.
- Pre-exposure Prophylaxis: Individuals without an established HIV-1 infection should take one tablet of Tenvir EM daily. To achieve maximum protection, it’s recommended to start the regimen 20 days before potential exposure to HIV and maintain it daily thereafter.
Contraindications and Precautions
Before starting Tenvir EM:
- Ensure that individuals meant for PrEP are not HIV-1 positive.
- Screen for chronic hepatitis B, as exacerbations might occur post discontinuation of Tenvir EM.
- Periodic renal function monitoring is advisable, given Tenofovir’s potential impact on kidney health.
Side Effects
While Tenvir EM is generally well-tolerated, some individuals might experience:
- Nausea or upset stomach.
- Fatigue or dizziness.
- Skin rashes.
- Changes in bone mineral density.
- Kidney or liver function abnormalities.
Interactions
Tenvir EM’s efficacy can be altered if consumed with certain drugs. Therefore, a comprehensive medication review is paramount before initiation. Some notable interactions include:
- Antiviral agents like acyclovir or ganciclovir.
- Antiseizure medications.
- Medications affecting renal function.
Storage
Tenvir EM tablets should be stored at room temperature, away from direct sunlight and moisture. Keep out of reach of children.
Conclusion
Tenvir EM, with its dual-action compounds Emtricitabine and Tenofovir disoproxil fumarate, is a game-changer in the world of antiretroviral treatments. Its efficacy in both treating and preventing HIV-1 infection underscores its significance in modern medicine. As with any medication, adherence to dosing, regular monitoring, and a thorough understanding of its pharmacology is crucial for optimal outcomes.

Reviews
There are no reviews yet.